PHOTONS AND LIGHT
![]()
Why is light important?
The interaction between light and matter is important to all aspects of physics and especially so to astronomy. Astronomers obtain all but a tiny fraction of their information about the universe in the form of electromagnetic radiation and it is only by understanding exactly how it interacts with the matter in the universe that we can hope to accurately determine the physical properties of the objects we see. To be able to do this we need a model which is capable of describing the behaviour of electromagnetic radiation in an entirely self-consistent fashion and such a theory has been surprisingly difficult to obtain. It is usually fairly easy to describe any single particular phenomenon but it is self-consistency which has been hard to provide. It is this strange behaviour of light and attempts to understand it that have been crucial to the development of the world of quantum mechanics.
![]()
How have theories of light evolved?
Historically - until around 300 years ago, scientists viewed light as a stream of particles with each individually carrying a small amount of energy. However, as more and more experiments with light were being performed it became apparent that this corpuscular model was unable to describe many of light's properties - diffraction, refraction and so on, and it eventually became clear that light was better described as a wave phenomenon. This view of light was strengthened when James Maxwell put forward a working theory as to the nature of these waves being oscillations of both the electric and magnetic fields - hence the name electromagnetic radiation.
However, at the beginning of this century it became clear that there were other aspects of light's behaviour, such as the photo-electric effect, that could not be described by the action of waves and it was necessary to re-invoke the particle theory of light. Both wave and particle descriptions are necessary to describe how light interacts with matter and the most appropriate visualization is dependent on the exact situation involved, this strange property is known as wave-particle duality. When we consider waves we envisage light as oscillations in electric and magnetic fields. When we consider the particle nature we view light as a stream of individual packets, or quanta, of energy. Each individual packet is called a PHOTON.
![]()
What is the photo-electric effect?
The photo-electric effect is the name given to the phenomenon whereby metals eject electrons in response to light falling on their surface. Because of the way the different atoms link together in a metal some of the electrons are largely free to move around the atomic lattice. These electrons are not strongly attached to individual atoms and are what give metals their properties. The electrons can easily be moved by the application of an electric field and this is what makes metals conduct electricity. Also, if the electrons are given extra energy by heating one end of a metal bar they are free to carry to energy down the bar, this is what makes metals good conductors of heat. If enough energy is given to a particular electron it can escape the metal altogether and move off into free space. This energy can be given to the electrons in a number of ways and one of which is by the absorption of energy carried by light which is shone on the metals surface.
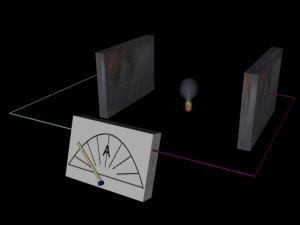
As the metal plate is illuminated electrons can absorb enough energy to leave the surface. They are accelerated across the potential difference supplied by the battery of cells and current flows in the circuit. The current is registered by the ammeter and when the light source is removed the current stops.
Please move mouse over image.
![]()
What do experiments show about the photo-electric effect?
If light of a certain frequency (monochromatic) illuminates a metal surface and the kinetic energy of the ejected electrons measured a number of very important facts emerge.
(1) It does not take longer for electrons to be ejected when the incident intensity is much reduced, there are simply less of them and increasing the intensity of incident light increases the number of ejected electrons but not their average kinetic energy.
(2) Below a certain frequency threshold (which is dependent on the metal) there are no ejected electrons even with very intense illumination.
(3) Above this frequency threshold the average kinetic energy of the ejected electrons increases linearly with increasing frequency.
![]()
Why are these observations so important?
It is these facts which make the photo-electric effect impossible to explain by considering light purely as a wave phenomenon and they also tell us about how the energy of photons is determined.
It is clear from (1) that the energy is not continuously supplied to the electron as would be the case with wave transport but that the electrons are receiving energy in fixed packets.
The electrons need a certain amount of energy to escape the metal, this is known as the work function, and with this in mind point (2) tells us that the energy they receive is in some way related to the frequency of the incident light.
Observation (3) confirms that the energy is linearly related to frequency.
The importance of photo-electric effect is highlighted by the fact that it is for work in this area that A. Einstein won the Nobel prize.
![]()
How is a photon's energy determined?
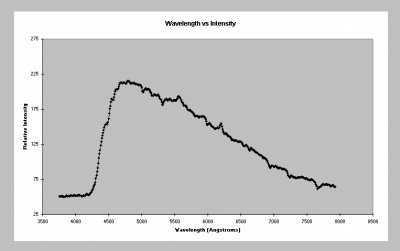
Another problem with the wave concept of light is in explaining the radiation given out by stars and other hot bodies. A good way to generate light is simply to heat an object. This is how a tungsten filament light bulb works. If we heat an object more and more we can see its colour change as it moves from being invisible to red hot to white hot. The gas at the centre of galaxy clusters and on the surface of stars where matter is accreting from a companion star the temperatures can be sufficiently hot that most of the radiation is emitted at X-ray wavelengths and XMM will be able to study these in detail. The spectrum of radiation depends on the temperature and the overall characteristic profile is known a black body curve. In fact when a light bulb is switched off it still radiates by virtue of it being at room temperature however the radiation is at wavelengths we are unable to see. The exact spectrum of the radiation emitted by a body because of its temperature cannot be explained if the energy is being radiated as a wave. However, M. Planck demonstrated how by supposing the energy was released in fixed quantities the problem could be solved. He called these fixed quantities of energy quanta and each quanta of energy is carried away by a photon. The energy of a photon (E) is linearly related to the frequency of the light (v) and the constant of proportionality is known as Planck's constant (h) such that E=hv. Planck's constant is around 6.626*10-34 Js.
This blackbody curve was produced by analysing the spectrum produced by the sun.
Click on picture for a higher resolution image
![]()
So, is light energy transported by a wave or a particle?
There is no single answer, it is impossible to describe all of the observed characteristics of light with just one or other model. Sometimes light exhibits wave like characteristics such as interference, diffraction and refraction and in other situations its particle like characteristics become apparent, such as in the photo-electric effect and in describing black body radiation. Whilst this might seem as though we do not have a coherent understanding of light this curious dual nature of radiation is an important and fundamental component of the physics of quantum mechanics. Light is not transported by one or other mechanism but is simultaneously transported by both and it is only when we perform observations or experiments that particular characteristics become apparent. Wave-particle duality, for particles as well as photons, is a necessary component of quantum mechanics which is currently the most successful theory we have for describing the behaviour of light and matter.
The End of the PHOTONS AND LIGHT topic.