


PARTICLES - THE CONSTITUENTS OF THE ATOM
![]()
The XMM detectors respond not only to X-rays, but also to the high energy electrons and protons in cosmic rays. These events have to be recognised and eliminated. For the same reason XMM cannot operate in the Earth's radiation belts, where the particle bombardment is very intense, so its orbit was chosen to make allowances for this.

The corruption in the lefthand image is due to cosmic rays bombarding the CCD camera. In order to remove it, image restoration techniques were used - see XMM Laboratory for more information.
![]()
What are atoms?
The name atom comes from the Greek word atomos meaning uncutable. For a long time atoms were considered the fundamental building blocks of the universe from which all matter was made. It was supposed that each of the different elements were simply different types of atom and they were completely stable and unchanging.
![]()
What happened to this view?
In 1897 J. J. Thomson demonstrated the existence of the electron. For several decades physicists had been unsure as to the exact nature of Cathode Rays - the name given to the phenomenon responsible for the glow seen in low-pressure gases when an electric field was applied, these devices were similar to today's Sodium lamp street-lighting.
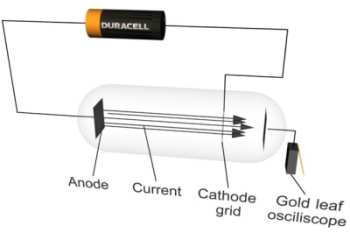
By applying a very large voltage between two plates it is possible to pull electrons across the gap. These electrons can strike gas atoms in the tube which are then excited and give off light as they subsequently decay back to their normal state. If the electrode to which the electrons are attracted has gaps in it some of the electrons can pass through and be collected on a gold leaf electroscope.
By collecting these so-called cathode rays on electroscopes and experimenting with magnetic fields Thomson determined that they were comprised of particles carrying electric charge. It is difficult to measure independently the mass (m) or charge (e) of particles but Thomson could fairly easily determine the ratio of these two parameters by closely observing their motion in magnetic fields. He found that for the cathode ray particles e/m was around 1800 times larger than the biggest ratio previously found, which was for the Hydrogen ion.
He concluded that these particles were a new form of matter and that they were present inside all atoms. They are now known as electrons and were the first identified sub-atomic particle. It is this discovery which signalled that atoms were not fundamental but had internal structure.
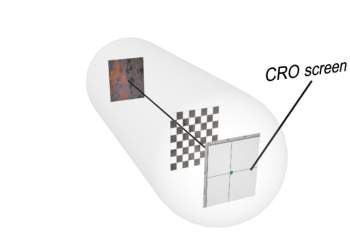
A force will act on a stream of electrons (a current) in the presence of a magnetic field. This shows electrons have a charge. If a magnetic bar is placed near a cathode ray oscilliscope, the spot on the screen will move.
Please move your mouse pointer over the image.
![]()
What did the electron's discovery mean about the rest of the atom?
Atoms were known to be overall electrically neutral and so it was clear that there must also be positively charged constituents to counteract the negative charge of the electrons. To try and probe the distribution of this part of the atom Ernest Rutherford performed experiments with newly discovered alpha particles. These are emitted in some forms of radioactive decay and are identical to ionized Helium atoms. They are a good source of fast moving and charged particles which makes them ideal for studying the electric charge distribution in atoms.
In 1911 Rutherford targeted a thin gold foil with these particles and observed the angles through which they were scattered. If there were a smooth distribution of positive charge within atoms one would expect the beam of alpha particles to become slightly blurred. However, Rutherford found a surprising result. Whilst the trajectory of most of the alpha particles was largely un-affected, they were sometimes scattered by very large angles and on occasion scattered back in the direction from which they came. To scatter through 180 degrees the fast moving alpha particles must have come to a halt before turning around and at this time all of their kinetic energy has been converted to electrical potential energy. The potential energy stored by two charges qalpha and qatom when separated by a distance r is
PE=(qalpha*qatom)/(4*pi*E0*r)
Where E0 is the constant known as the permittivity of free space.
For there to be somewhere in the atom where this potential is high enough to stop the relatively energetic incoming alpha particles, the positive charge in the nucleus has to be packed very tightly such that the ratio qatom/r is large in some places. By studying the exact distribution of the scattering Rutherford was able to show that the atomic nucleus was extremely tiny. A typical atom is around 10-10 m in diameter whilst a typical nucleus is only around 10-15 m in diameter. If an atom were the size of a football stadium the nucleus would be like an ant in the middle of the pitch. Incidently on these scales the electrons would whizzing around the very edge of the stadium with a size at least 1000 times smaller than the ant.
The particles responsible for this tightly packed positive charge were named protons and were another entirely new sub-atomic particle. Each proton carries the same magnitude electric charge as the electron, although positive rather than negative, but is almost 2000 times more massive. In its natural state an atom contains the same number of protons as electrons and it is this number which determined what type of atom it is. Hydrogen contains one of each, Helium two, Lithium three and so on
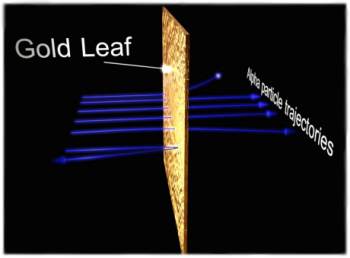
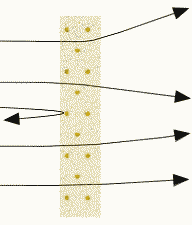
Alpha particles are directed towards a target of gold foil. Whilst the gold foil is overall electrically neutral as there are the same number of electrons as protons their distribution within the target are very different. The deflections in the alpha particle trajectories as they are repelled by the positive charge distribution within the atom provides information on the actual charge distribution. The ability for the gold foil to scatter some alpha particles through 180 degrees is evidence for an extremely compact and highly localized charge distribution.
![]()
Is this the complete picture?
No, it became clear that there was still something missing and that the nucleus had to contain extra new particles. Atoms of the same element can sometimes have different masses, so called isotopes of the element, and there must be other particles responsible for these different mass isotopes. It was also seen how bombardment with alpha particles could sometimes cause the ejection of some unknown and uncharged particles from within atoms. Finally, in 1932, James Chadwick, again using alpha particle scattering techniques, was able to consider in more detail the structure of nuclei and demonstrated the final atomic constituent at these scales - the neutron. These are similar in mass to the proton but are uncharged and there can be a variable number of them in atoms of the same element. Not all of these isotopes will be stable or occur naturally in nature and as a rule of thumb there are generally a similar number of, or slightly more, neutrons than protons.

Elements can have a different number of neutrons. The chemical properties stay the same but the atom mass differs. In the above picture there is a hydrogen, deuterium, and tritium atom.
![]()
What is the current classical view of the atom?
The classical view of the atom now includes these three particles: ELECTRONS with a charge of -1.602*10-19C and a mass of 9.110*10-31kg define the size of the atom as they orbit a the compact nucleus. This nucleus contains both PROTONS, with a charge of +1.602*10-19C and a mass of 1.672*10-27kg and NEUTRONS with zero charge and a mass of 1.675*10-27kg. Collectively the protons and neutrons are called NUCLEONS. It is currently believed that the electrons are truly fundamental particles but that the nucleons are themselves constructed from still smaller particles called quarks. There are two types of quark which between them make up the protons and neutrons. Both nucleons contain three quarks, protons contain two of one sort and one of the other whilst neutrons contain one of the first kind and two of the second.

