


The spectra of atoms contain signatures of the chemical elements which are present even in the most distant parts of the Universe. XMM will look for these signatures in the sources which it observes with the CCD's or the grating spectrograph.

IMAGES - A CDD (left) and a grating spectrograph (right) which are used in the XMM satellite.
![]()
What is the current classical view of the atom?
The classical view of the atom now includes these three particles: ELECTRONS with a charge of -1.602*10-19C and a mass of 9.110*10-31kg define the size of the atom as they orbit a the compact nucleus. This nucleus contains both PROTONS, with a charge of +1.602*10-19C and a mass of 1.672*10-27kg and NEUTRONS with zero charge and a mass of 1.675*10-27kg.
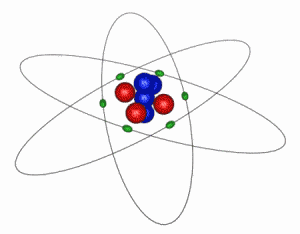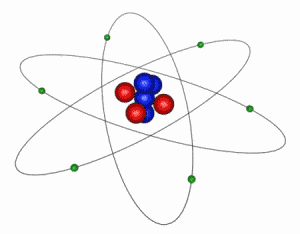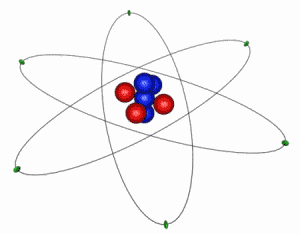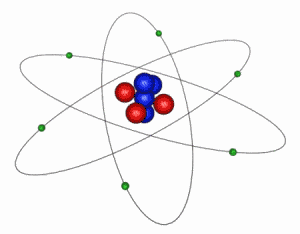
IMAGE - The Classical Atom
This is an extremely simplified and idealized idea of how an atom looks. The electrons (green) orbit around a compact nucleus containing protons (red) and neutrons (blue). In this diagram the nucleus is shown 5000 times larger than is should be in comparison to the electron orbits. The electrons' are even more exaggerated and in reality are at least 1000 times smaller than the nucleus.
Collectively the protons and neutrons are called NUCLEONS. It is currently believed that the electrons are truly fundamental particles but that the nucleons are themselves constructed from still smaller particles called quarks. There are two types of quark which between them make up the protons and neutrons. Both nucleons contain three quarks, protons contain two of one sort and one of the other whilst neutrons contain one of the first kind and two of the second.
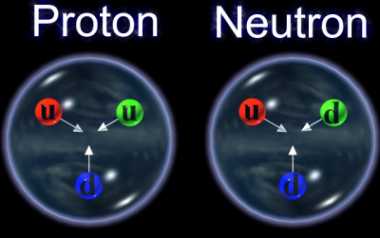
IMAGE - A proton and a neutron
It is believed that nucleons are made up of quarks. Two up and one down quark make a proton and two down and one up quark create a neutron.
![]()
What are the most important parts of atoms?
Each component dominates in different ways. The nucleus is most important to the mass of the atom, it defines which element it is and also determines its stability against radioactive decay. The electrons, on the other hand, dominate the interaction of atoms with the rest of the universe - both their association with other atoms and the vast bulk of the interaction between light and matter. However, in terms of what is actually present when we consider an object it is the empty space which dominates. When we hold a brick only one thousand million millionth (10-15) of its volume is anything other than empty space. When large stars run out of the fuel needed to maintain their temperature and hence gas pressure they collapse under gravity. If the star is massive enough the gravitational force can compress the atomic nuclei together and largely remove the empty space. The entire star becomes as dense as the nuclei and is known as a neutron star. A house brick made of this material would weigh the same as Mt Everest.
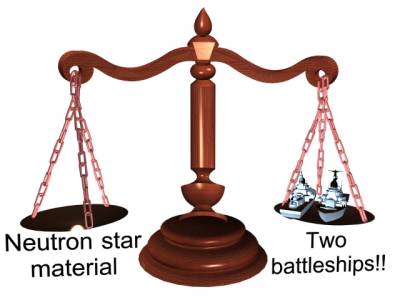
IMAGE - a pinhead of neutron star material would weigh the same as two large battle cruisers!
![]()
What do we mean by the atomic number of an atom?
The number of protons in an atom determines which particular chemical element it is and is known as the atom's atomic number. For example Carbon always contains 6 protons. That is what makes it Carbon. If it had 5 protons it would be Boron and if it had 7 it would be Nitrogen. A neutral atom contains the same number of electrons as it has protons. However, it can be easy to remove one or more electrons from an atom such that it develops a net electric charge. The process of removing electrons is called ionization after which the atom is referred to as an ion. For instance a Carbon atom with two electrons removed is known as a twice ionized Carbon ion. Electrons can also be added to an atom to make negative ions but these are usually unstable since it can be hard to make the extra electron stick.
![]()
What do we mean by the atomic weight of an atom?
The mass of an atom is contained largely within the nucleus. Neutrons and protons have roughly the same mass which is known as the atomic mass unit. The atomic weight of an atom is close to the number of nucleons it contains. The atomic mass unit is defined such that a Carbon atom containing 6 neutrons in addition to its 6 protons has a mass of exactly twelve atomic mass units (this particular isotope with 12 nucleons is known as Carbon-12). However, because of the way in which nuclei are held together the atomic weight doesn't have to exactly be a whole number. The energy involved in the binding together of the nucleons effects the mass of the atom and is known as the mass deficit. This stems from Einstein's famous equation E=mc2 and means that particular arrangements of nucleons can have effective masses which are slightly different from the number of nucleons they contain multiplied by atomic mass unit. For instance there is a form of Carbon which contains 8 neutrons in addition to the 6 protons. This isotope of Carbon has exactly 14 nucleons (and is known as Carbon-14) but its atomic weight is 14.003242 atomic mass units.

Albert Einstein showed at the beginning of the 20th century that matter can be converted into energy (eg nuclear fusion reactions in the Sun).
Please put your mouse pointer over the image
![]()
What holds an atom together?
We know the solar system is held together by a balance between the centripetal force due to the planetary orbits and their gravitational attraction towards the Sun. The classical view of the atom is like a miniature Solar system but what are the forces involved?
The classical view of the atom is like a miniature Solar system.
Please put your mouse pointer over the image
On the scale of atoms the gravitational force is not strong enough to hold the electrons to the nuclei and whilst gravity is the dominant force on the scales of humans, the solar system, the galaxy and the universe as a whole, it is insignificant on atomic scales and it is the electric force between the positively charged nucleus and the negatively charged electrons which hold the electrons in orbit. However, while the electric force holds our electrons in place it gives us a problem for holding our nucleus together. The nucleus contains very closely packed positively charged protons and the electric force causes them all repel each other. Again gravity is not strong enough to hold them together and there must be some other force at work within the nucleus. This force is responsible for the binding together of nucleons and controls their stability, it is known as the strong nuclear force.
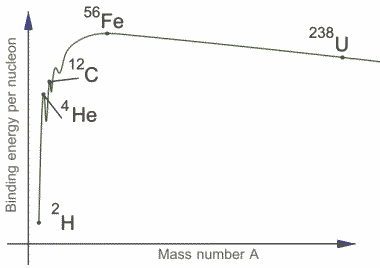
IMAGE - In a graph of Mass Number against Binding Energy per Nucleon it can be seen that the most stable element is 56Fe because the binding energy per nucleon is greatest.
![]()
Why are electron orbits stable?
The classical view of electrons orbiting the nucleus creates problems with explaining the stability of these orbits. By moving in a circular path the charged electrons are continually being accelerated and this should make them a source of electro-magnetic radiation. If the electrons were to be continually radiating electro-magnetic waves they would be losing energy and their orbits would decay until the electrons fell into the nucleus. Niels Bohr first addressed this in 1913 when he argued that electrons could exist in certain fixed orbits without radiating and were therefore stable against collapse but he couldn't explain why such stable orbits arise. Later Louis de Broglie explained their existence by suggesting that electrons (and all other forms of matter) could be considered both as either a wave or a discrete particle, the so called wave-particle duality. The wavelength of these de Broglie waves are determined by the momentum of the electrons and only those orbits for which there are a whole number of wavelengths in the circumference can exist.
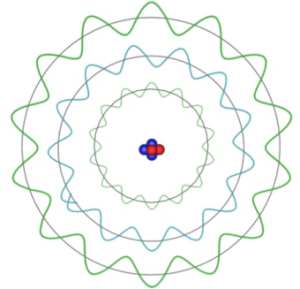
The electron must have a particular angular momentum to maintain a particular orbit. In de Broglie's theory this momentum determines the electron's wavelength and only those orbits for which an integer number of wavelengths fit into a circumference can exist. In this diagram both of the green orbits satisfy this condition but the blue one does not and cannot exist. In classical mechanics the outer orbit would radiate energy and so decay towards the nucleus, with quantum mechanics since the electron cannot exist in the blue orbit it is held in the outer orbit and does not radiate.
![]()
Does this mean electrons do not interact with electro-magnetic radiation?
No, but it requires another quirk of quantum mechanics. While electrons are orbiting the nucleus they do not radiate. However, it is possible for the electrons to move from one particular orbit to another (so long as there aren't already too many other electrons in that orbit) if they do it quickly. If they are to move to a higher orbit they must absorb energy and if they are to move to a lower orbit they must lose energy. This energy transfer can happen in different ways such as in collisions with other atoms or electrons but the most interesting mechanism is that which involves light. By absorbing energy from light atoms electrons can move to higher orbits and the atom is said to become excited since it contains more energy than the most stable configuration. Similarly electrons in excited states can fall to a lower orbit and emit radiation. Because only certain electron orbits can exist there are only certain quantities, or QUANTA, of energy that can be exchanged in this way and this gives the radiation from any particular atomic species very specific characteristics. These allow us to determine the type of radiating atom simply by measuring the particular energies of the radiation it is emitting or absorbing. This works no matter how far away the atoms are and is how astronomers determine the elements within distant objects.
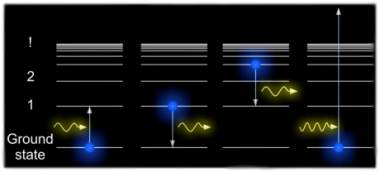
We can describe the allowed orbits of electrons by the energies associated with them. This diagram indicates the particular energy levels associated with a Hydrogen atom and some of the different transitions which its single electron can make. The lowest energy state is the known as the ground state. The next highest energy state is known as the first excited state and so on. The energy difference between states rapidly gets smaller and converge to a particular value, if the electron obtains more energy than this value it is no longer considered part of the atom and becomes ionized. In diagram A an atom is raised to its first excited state by absorbing a photon of just the right energy. In diagram B an excited atom decays back to the ground state by emitting a photon of the same fixed energy. However, there are many other possible transitions and in diagram C the atom emits a lower energy photon in moving from the third excited state to the first excited state. The atom can interact via a whole family of fixed energy transitions but above a certain energy the electron is ripped from the atom and becomes ionized, as in diagram D. Because it is no longer constrained by fixed orbits the electron can have any arbitrary energy and so at energies beyond the point at which it becomes ionized the atom can interact with a continuous range of energies both in absorption or emission when a free electron is captured by a previously ionized atom.

