


How does latent heat affect our everyday lives?
There are many ways in which latent heat affects us but arguably the most important is through the weather. Water is continuously changing its state; ice melts to form water, water is vaporised to form clouds. A sort of sublimation also occurs when water vapour comes into contact with a very cold surface; it freezes directly (frost). This is not real sublimation because atmospheric pressure is greater than that equivalent to the triple point. There is simply not enough time for the condensed vapor to come together on the surface to form sufficient liquid which can then freeze to form ice.
Weather is responsible for the transport of heat from the hot side of the Earth to the cold side and since latent heat is stored energy this energy can also be transported. The weather is a mechanism that attempts to "even out" differences in temperature and pressure. In some situations however the release of latent heat increases these differences rather than decreases them and self-propagating storms can result. In their most extreme form these are the tornadoes which are extremely powerful and can wreak enormous damage.
![]()
What has latent heat got to do with XMM?
It is not so much the energy content of latent heat as the change of state which is of significance to XMM and its effects are very subtle and very significant.
The CCDs on XMM require to be cooled to very low temperatures (-90o C to -130o C) and therefore the CCDs themselves, as well as the cryostats which are cooling them, provide cold surfaces which are able not only to cool gases and vapours which come into contact with them but also to remove their latent heats so that the vapours become trapped on these cold surfaces. Because of the very low pressures that occur in space the process is one of sublimation. Hence cold surfaces act as "vacuum pumps" pumping out the volumes in the spacecraft and building up layers of the trapped gases on the cold surfaces (vacuum pumps known as cryopumps work on this principle). The thickness of such layers is not very great; indeed it would not be possible to see this "frost" with the naked eye. But even thicknesses of several monolayers of condensate (a few atoms thick) can be significant.
Where they affect XMM is on the performance of the CCDs and on the performance of the thermal system. Cryodeposits of water and hydrocarbons on the surface of the CCDs causes a deterioration in the response of the CCDs to X-rays and they become less good detectors. Cryodeposits of water, in particular, on a polished surface will make it appear less shiny, significantly so in the case of infrared radiation (heat). Shiny surfaces are used to provide good heat insulation to inhibit heat flow and the effectiveness of such surfaces is affected when trapped contaminants are present. In 'physics speak' the emissivity of the surface is increased.
This is not a trivial problem. Gases which can readily be trapped even on warm surfaces at atmospheric pressure (i.e. on the ground) can desorb or out-gas when the pressure is reduced (i.e. in space). Many kilograms of water can be carried in the carbon reinforced plastic (CFRP) which makes up the large, main telescope tube of XMM which then comes out in space. Designs are adopted to ensure that such gases can quickly vent to space and thus disperse before coming into contact with cold surfaces, but no system is perfect and we cannot rely solely on this.
![]()
How does the CCD de-contamination system work on XMM?
Fortunately there is a very simple remedy; if the temperature can be raised to a suitable level the contaminants can be driven off. And because the ambient pressure is low (a) the temperatures required to do this are not very high and (b) the contaminants can be sublimed off directly. Hence ice can be vaporised at about -70o C and the hydrocarbons at about 0o C. The higher the temperature above these threshold temperatures the faster will the contaminants be driven off so in effect it is controlled by the wattage of the decontamination heater. On XMM such heaters are typically between 5 and 10 W. Hence, when necessary, the heaters will be turned on for short periods to "clean up" the system.
Conversely, the colder a surface the greater is the rate at which they will re-contaminate. We expect to run the CCDs at about -90o C in the early part of the mission reducing the operating temperature closer to -130o C towards the end of the mission as the CCDs suffer damage from energetic charged particles. But as the mission progresses the amount of ambient water vapour will diminish as it slowly disperses into space so at all times we expect to have significant periods of time between the decontamination operations when the CCDs and other systems are working as required.
On the ground a similar scheme is used in cleaning bits of space hardware. They are put in a vacuum chamber and baked at temperatures typically in the range 60o to 100o C for several days.
![]()
What is a heat pipe and how does it work?
A heat pipe utilises latent heat and the change of state to transport heat very effectively from one end of the pipe to the other. It is so effective that even for large amounts of heat flow there is only a very small temperature difference between one end of the pipe and the other. They are designed to operate at temperatures very close to the temperature of vaporisation of the working fluid in the pipe.
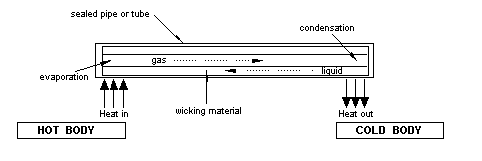
At the "hot" end of the pipe the liquid is vaporised by absorbing heat from the thing to which that end of the pipe is attached. There is a build up of pressure which drives the gas to the other "cold" end of the pipe where it liquefies giving up its latent heat to the thing to which that end of the pipe is attached. The pressure falls and the liquid can be returned to the other end of the pipe by capillary action through a wicking material. The pipes have to be operated in a free fall system (i.e. in space) so that the force of gravity does not come into play. This then enables the small pressures generated to drive a circulation and, because the phase changes occur at a given temperature, the pipe is effectively an isothermal element.
The 'effective thermal conductivity' to 'mass' ratio of a heat pipe is vastly superior to that of even copper. The amount of heat they can transfer is not dependent on the length of the pipe and can therefore be used to transport heat between parts of structures which are metres apart without placing excessive demands on the mass budget. Unfortunately they are expensive and only operate at the given vaporisation temperature of the medium.

