


Why is temperature important to XMM?
Temperature is vitally important to all of us. If , through lack of control, our body temperature goes outside what are very narrow limits, we cease to function, we die. Similarly XMM. All the subsystems have specifications defining the temperature range within which they will perform their functions satisfactorily and for which they have been tested. If the thermal design of the spacecraft is inadequate, or is not robust enough to survive changes in environment then the spacecraft dies.
The aim of a good thermal design of a spacecraft or a space experiment is to ensure that the temperatures of the various parts are not extreme and certainly not outside the temperature range over which components such as electronics are meant to operate. In general it is convenient to design things such that the mean temperature is somewhere in the range 10oC to 30oC because this is typical of temperatures on the surface of the Earth and hence temperatures at which equipment operates well and for which experiments have been calibrated. Many spacecraft carry detectors of one sort or another and in general these detectors are best run at a cold temperature; hence they require their own thermal design. For example, the detectors on XMM which are called Charged Coupled Devices (CCDs) need to be operated at a temperature between -90oC and -130oC.
![]()
What is our perception of temperature?
We all have a 'feel' for temperature simply because we feel it as degrees of hot and cold. Our normal body temperature is controlled at a value of about 40oC, which is higher than most of our surroundings and we use it as a reference for feeling hotness or coldness. So a bath at 60oC feels hot and the sea at 20oC feels cold. However, in terms of what we feel, it must be a little more complicated than this because I feel warm when I am at home in air which is at 20oC, yet I feel cold if I stick my head out of the window of a moving car on a day on which the air temperature is 20oC. Why? Also if I pick up a piece of metal at 20oC it feels cold, and if I pick up a piece of polystyrene at 20oC it doesn't feel cold. Why?
Because our temperatures are controlled to be above normal ambient temperatures, human beings belong to the class of warm blooded animals. Animals who do not have such systems are called cold blooded. Yet both systems work because both species have survived pretty well. Why might this be so and what has it to do with spacecraft?
![]()
What is the physical meaning of temperature?
The physical meaning of temperature is rather more precise and at the same time rather more esoteric. It is to do with how the energy of a system of 'particles' is distributed amongst the individual 'particles'.
Consider a certain amount of energy being dumped into a system of particles. The particles redistribute (or share) the energy through collisions with each other and after some time come to a state where the distribution of energies amongst the particles is unchanging and the system is described as being in equilibrium. This unchanging distribution has a standard, characteristic shape (see diagram below) which can be predicted theoretically and which only requires one parameter to describe it. This parameter is the temperature. A given particle can still change its energy but at the expense of some other particle since the total numbers of particles with any given energy will remain constant with time. If we now put more energy into the system, this will also be redistributed giving us a similar shaped distribution but described by a temperature which now has a higher value. For more click here.
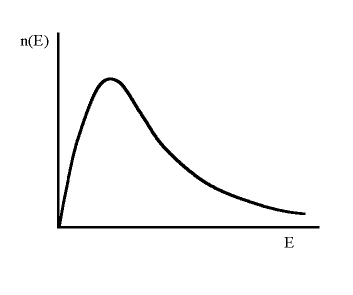
The precise distribution depends on the type of particle. For example, particles on the scales of atoms and greater (so called classical particles) have an equilibrium distribution called a Maxwell-Boltzmann distribution, whereas photon 'particles' have a Planck distribution (or blackbody distribution). Particles on scales smaller than the atom (quantum particles) have yet another distribution. However, the temperature which describes the different distributions is still the same; it is not some different type of temperature.
A moot point is that the Universe is not in a state of equilibrium so how can we talk meaningfully of temperature? Although this is strictly true we can none-the-less make the equilibrium assumption in many cases and use the concept of temperature to describe a whole lot of physics in a very satisfactory way.
![]()
How can we deal with temperature in a practical way?
For practical purposes we only need to know two things at this stage. Firstly, if we put a net amount of energy into a system its temperature will rise. Secondly, heat will always flow from a hotter body to a cooler body.
These two statements tell us rather different things. The first tells us that we can use temperature to quantify the amount of energy stored by a body. We know that it depends on the specific heat and the mass of the body as well as its temperature. The second tells us something far more subtle in that temperature is indicative of the level of the energy; heat flows because of the difference in level irrespective of the amount of energy in each body.
We can see from this that temperature is inexorably linked with energy and therefore with heat flow. To understand heat flow we need to know about the mechanisms by which this occurs, namely conduction, radiation and convection.
![]()
What determines the temperature of a single body??
The magnitude of the temperature of a single body is determined by the flow of heat into and out of the body. If it receives energy at a faster rate than it disposes of it, it will heat up. Conversely if it looses heat faster than it gains heat its temperature will fall. These transient changes in temperature will continue until the 'in' 'and' out heat flows are in balance after which the magnitude of the temperature remains steady.
![]()
What determines the temperature of two bodies in a thermal system??
A hotter body looses energy to a cooler body by one of the three mechanisms above thereby loosing some of its internal energy. If the body cannot replenish its internal energy with a heat source the temperature of the body will therefore decrease. This continues until the temperature of the two bodies is the same and there is then no net flow of energy between them.. The components are then said to be in thermal equilibrium.
Clearly the Universe is not in a state of thermal equilibrium because we only 'see' the Universe by virtue of an unequal exchange of energy.
To maintain a steady flow of heat between two bodies (i.e. to maintain the temperature difference (gradient)) therefore requires the hotter body to have an internal source of energy; i.e. it has to have some internal heat dissipation. In this situation we speak about a state of dynamic thermal equilibrium, or simply thermal steady state, where the net heat flows are steady and the consequent temperatures are different to each other though unchanging.
A different insight into these concepts can be obtained from consideration of water in a bath. If you want to explore this further click here.
![]()
How are temperature gradients calculated?
The rate at which heat flows between one body and another (i.e. the power) depends on that agent which is supporting the heat flow and that agent which is opposing the heat flow. The first is clearly related to the temperature gradient because without a gradient there is no heat flow. The second relates to the thermal resistance between the bodies; if the resistance is infinite then again there will be no heat flow.
An interesting analogy is the flow of electricity through a wire, or, the flow of a fluid through a pipe. To explore this further click here.
In general terms we can see therefore that the temperature gradients are in some way proportional to the power and in some way inversely proportional to the resistance. We can expect the functional relationships to be different for the three different transfer mechanisms.
As an example consider the human body. We have an internal supply of energy which we maintain through eating. Notwithstanding the various mechanisms the body has for maintaining our body temperature nearly constant, when we feel cold we put on more clothing which resists the loss of heat flow; the clothing acts as a thermal insulator and consequently we feel warmer.
![]()
XMM will be in space and "space is cold" so will XMM also be cold?
We tend to talk of space being cold but we need to be clear what we are talking about. The Earth is in space but the Earth is not cold; the surface of the Earth is at a temperature of about 20oC.Why is this? Does the Earth have an internal supply of heat which keeps it warm or is it warmed by something else.
Obviously the Earth is warmed by the Sun (although the Earth's core is very hot it is not effective in heating the surface. Why?).
Space is only cold in places far removed from stars.
![]()
Why is the Earth at about 20oC (293oK)?
As discussed above, the temperature of a body is determined by a consideration of the inflow and outflow of heat. The Earth essentially receives heat only from the Sun (i.e. from a disk in the sky which is 1/2 o in diameter and at a temperature of 6000o K) and looses heat in all directions to cold space (which we can take as almost 0o K). See if you can show by calculation from this that the Earth's temperature is what we observe it to be. If you need help click here.
![]()
Why then is the Earth only slightly cooler at night when it is not illuminated by the Sun?
This is so because the heat is very effectively transported from the hot side of the Earth to the cold side by the atmosphere. When this motion is coupled with the motion of the spinning Earth we get a circulation of air which we call the weather. Hot air rises and thus vertically transports energy by convection, and the heating and cooling of the atmosphere sets up regions of high and low pressure which then generates the winds hence distributing the warm air over the surface.
Air is not a good conductor of heat (hence double glazing). Similarly water is not either. Water transports heat in a similar way to air and we get vertical and horizontal flows of water in the oceans which we call currents. These are also very important in equalising temperatures on Earth and also affect the weather/climate (examples: Gulf Stream, Elnino)
Another reason is because the Earth spins about its axis (once in 24 hours) and there is insufficient time for the temperature on the surface to reach a steady value (i.e. even thermal steady state is not reached). So the temperature has a cyclical, diurnal variation If this diurnal variation is averaged out, can we then say we have thermal steady state?. The answer is essentially yes but you might like to think about this a bit more; if so click here.
![]()
Is the temperature of the Moon very different from that of the Earth?
The Moon is essentially at the same distance from the Sun as is the Earth so should it have the same average temperature? The answer would be "yes" were it not that the Earth's atmosphere acts as a thermal blanket which keeps the surface of the Earth warm. When there are clouds the atmosphere is an even better blanket (remember that it is colder on a clear night because heat is also lost from the surface of the Earth by radiation).
However the variation of the temperature over the Moon is much greater than that of the Earth because it has no atmosphere or oceans. Also it spins about its axis much more slowly (i.e. once a month).
The temperature on the hot side of the Moon is about +110oC and on the cold side about -180oC.
![]()
What has all this got to do with spacecraft in general and XMM in particular?
A spacecraft in low Earth orbit is also at a similar distance from the Sun as is the Earth and the Moon and could therefore be expected to be at much the same temperature. It is perhaps a bit more similar to the Moon because it does not have an atmospheric blanket (indeed convection as a mode of heat transfer does not feature in the thermal design of spacecraft). One difference however is the presence of the Earth itself which acts as an additional heat source. It reflects some of the Sun's radiation from the tops of the clouds back into space on the day side. It is also a warm body at about 20oC in its own right and radiates this energy back into space from the top of the atmosphere both on the day and night side (the effective temperature at the top of the atmosphere on the upper side of its "blanket" is about -20oC). Some of this radiation is intercepted by the spacecraft in addition to that which it receives directly from the Sun.
We can therefore surmise that the thermal environment of a satellite overall is about the same or slightly warmer than that of the Earth itself. Hence there is not generally a problem with the overall power budget. What a thermal design has to do therefore is to ensure that temperatures are generally equalised as well as designing for those components which require special temperatures (e.g. detectors).
In short we want to make our satellite more like the Earth than the Moon in terms of the range of temperatures, though we must recognise that the convection mode of heat transfer which effects this on Earth is not an available mechanism on a satellite.
![]()
How do we control conduction in the design?
Conduction is about the transfer of energy by collisions between one particle and another. Consequently matter in any of its forms (gas, liquid, solid, plasma) will conduct heat to a lesser or greater extent. In general it is more effective in situations of higher density so we could expect solids to be good conductors. This is clearly not the whole story since we know that it is the metals which are the really good conductors rather than the non-metals which are insulators (this is why saucepans usually have a non-metallic handle).
Spacecraft have a solid structure to give it strength and rigidity so conduction will always occur. We control the effectiveness of the conduction through our choice of materials and by controlling their geometry. For example a short, thick rod of any material is a better conductor than a long, thin rod of the same material. However any design has to be consistent with the mechanical constraints and the mass constraints.
![]()
How do we control radiation in the design?
Radiation is also to do with the transfer of heat through collisions, but this time between particles and photons (packets of energy). The radiative heat coupling between different components can be controlled through the geometry of the radiating elements and the surface finish of the elements. For example a surface with a large area is a better radiator/absorber than one with a small area. Also rough surfaces are good radiators/ absorbers whereas shiny, polished surfaces are good reflectors which therefore do not absorb energy and so are not good radiators/ absorbers. Besides direct treatment of surfaces we can use paints and tapes which adhere to the radiating surface.
However to make a surface very insulating in the radiative sense we have to cover it with a blanket which is made up of multiple layers of very thin, very shiny foils made of plastic material very similar to that from which crisp packets are made. Indeed you may have seen marathon runners being covered in a single layer of such a foil at the end of the race. Often you will see pictures of spacecraft which have these multilayer blankets on the outside of them.
![]()
Are there any other ways of controlling spacecraft temperatures?
Yes; there are quite a large number of rather more sophisticated ways of controlling temperatures though perhaps not quite as elegant. These are so-called active heating and cooling devices which have to be electrically powered. A simple example of the former is a heater mat which operates like an electric fire but at a much lower power. An example of the latter is a cryogenic device which operates much like a conventional refrigerator.
![]()
How are the temperatures of the CCDs on XMM controlled?
Here we use a multi-stage, passive radiator to cool the CCDs to a very low temperature in combination with a local heater to actually control the temperatures to selected values above this. Electronics monitors the temperature and brings the CCDs to the required temperature by controlling the heater power.
The end of the Temperature topic.

