


![]()
The spectra of atoms contain signatures of the chemical elements which are present even in the most distant parts of the Universe. XMM will look for these signatures in the sources which it observes with the CCD's. or the grating spectrograph.
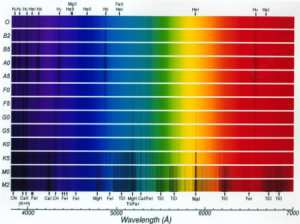
A typical spectrum
Please click on the image for a higher resolution image (please note is is aprroximately 100k in size!)
What are photons?
The concept of wave-particle duality asserts that all the constituents
of matter have both a wave-like nature and a particle-like
nature. Light waves have both an electric field and a magnetic field,
which oscillate as the wave moves forward. [This is why light is
often called electromagnetic radiation.] The energy of the light waves
is carried by its particles, the photons. Einstein suggested that if
the wave has a frequency v, then the photons would have an
energy hv where h = 6.626 x 10-34Js is called
PLANCKS CONSTANT. Both the wave and the photons move with the
same speed so that they keep in step.
The colour of visible light is determined by frequency - red light has
a lower frequency than blue light. Thus, the photons of red light are
smaller than those of blue light.
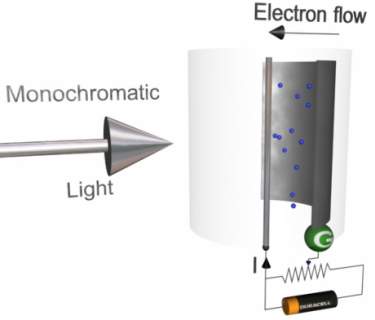
Using the above apparatus, it can be shown that light is made up from descreet packets of energy called photons.
![]()
What happens when atoms radiate light?
While electrons in an atom are orbiting the nucleus, they do not radiate. However it is possible for the electrons to move from one orbit to a vacant space in another provided that they do it quickly. If they move from a higher orbit to a lower one, the atom must radiate away the energy difference as a photon. Each atom has a set of different orbits or energy states, and each pair of these corresponds to a particular energy difference and so to a particular photon frequency hv. The set of frequencies which an atom can radiate gives it a characteristic signature which allows it to be recognised. This works no matter how far away the atoms are and allows astronomers to determine the elements of the distant stars or galaxies.
Does this mean electrons do not interact with electro-magnetic radiation?
No, but it requires another quirk of quantum mechanics. While electrons are orbiting the nucleus they do not radiate. However, it is possible for the electrons to move from one particular orbit to another (so long as there aren't already too many other electrons in that orbit) if they do it quickly. If they are to move to a higher orbit they must absorb energy and if they are to move to a lower orbit they must lose energy. This energy transfer can happen in different ways such as in collisions with other atoms or electrons but the most interesting mechanism is that which involves light. By absorbing energy from light atoms electrons can move to higher orbits and the atom is said to become excited since it contains more energy than the most stable configuration. Similarly electrons in excited states can fall to a lower orbit and emit radiation. Because only certain electron orbits can exist there are only certain quantities, or QUANTA, of energy that can be exchanged in this way and this gives the radiation from any particular atomic species very specific characteristics. These allow us to determine the type of radiating atom simply by measuring the particular energies of the radiation it is emitting or absorbing. This works no matter how far away the atoms are and is how astronomers determine the elements within distant objects. -->
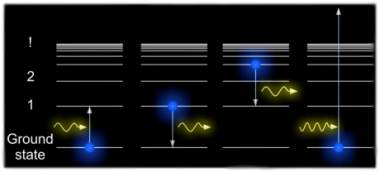
We can describe the allowed orbits of electrons by the energies associated with them. This diagram indicates the particular energy levels associated with a Hydrogen atom and some of the different transitions which its single electron can make. The lowest energy state is the known as the ground state. The next highest energy state is known as the first excited state and so on. The energy difference between states rapidly gets smaller and converge to a particular value, if the electron obtains more energy than this value it is no longer considered part of the atom and becomes ionized. In diagram A an atom is raised to its first excited state by absorbing a photon of just the right energy. In diagram B an excited atom decays back to the ground state by emitting a photon of the same fixed energy. However, there are many other possible transitions and in diagram C the atom emits a lower energy photon in moving from the third excited state to the first excited state. The atom can interact via a whole family of fixed energy transitions but above a certain energy the electron is ripped from the atom and becomes ionized, as in diagram D. Because it is no longer constrained by fixed orbits the electron can have any arbitrary energy and so at energies beyond the point at which it becomes ionized the atom can interact with a continuous range of energies both in absorption or emission when a free electron is captured by a previously ionized atom.
![]()
So what happens when light is absorbed?
Absorption of photons is just the reverse process to emission. For example, a photon in the beam of light is incident on an atom in the absorber; if the photon has just the right energy corresponding to the difference of the energy states or orbits of the atom the photon may be absorbed. Then the electron in the atom moves from its initial state to a higher one. Evidently the lines of a particular element in its emission spectrum occur at exactly the same frequencies as those in its absorption spectrum. Please click here for a JAVA animation.
An electron revolving around the nucleus may capture an incident photon. Once that occurs, the electron is raised to a higher energy level and thus changes its orbit to one with a larger radius. While being in the excited state, the electron re-emits the photon after some time and returns to the ground state. Note that one can observe the phenomenon involving the emission of the photon and its immediate recapture upon change of orbit (virtual photon emission).

