


Some radioactive sources emit x-rays at accurately known wavelengths. These are used in a special mode of the XMM detectors to calibrate their wavelength response.
![]()
What makes nuclei stable?
The protons in a nucleus strongly repel each other due to the electrostatic force but are held together by the attractive strong nuclear force which acts between nucleons in general and not just the protons. This means that by adding the uncharged neutrons we can increase the force that holds the nucleus together but do not add to the electrostatic repulsion. This explains why there are typically more neutrons than protons in stable nuclei. However, if this were the only factor in determining the stability it is clear that the most stable nuclei would contain enormous numbers of neutrons in comparison to protons.
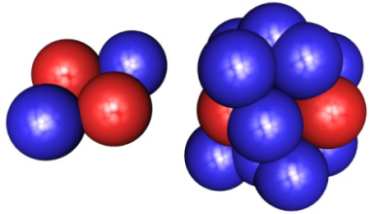
IMAGE - A helium atom with two neutrons (left) and a lithium atom (right) with quite a few neutrons! Is it possible to have this many neutrons and at the same time keep the nucleus stable?
However, there are certain rules to do with packing protons and neutrons together that come from quantum mechanics. One of these rules doesn't allow certain types of particles, called fermions, to be packed next to each other if they have the same properties. By this we mean that if two neutrons are in the same place they cannot have the same energy and angular momentum. This is known as the Pauli exclusion principle and since electrons, protons and neutrons are all fermions they are all subject to this rule.
Another result of quantum mechanics is that these fermions can only have one of two distinct angular momenta, they either spin clockwise at a fixed rate or anti-clockwise at the same rate. The consequences of the exclusion principle can be demonstrated by considering an analogy of building blocks. Suppose protons are red building blocks and neutrons are blue building blocks. For a nucleus the most stable configuration, ie that which has least energy, is for all of the blocks to be on the floor. However, the exclusion principle insists they cannot all have this same energy and that only two of each colour block can be on the ground - in the so-called ground state - the next pair of each colour must be stacked on top of this lower pair and so on. Each pair of blocks in the stack have the same energy but opposite angular momentum and the next level of blocks have the same angular momentum as the blocks below them but different energies.
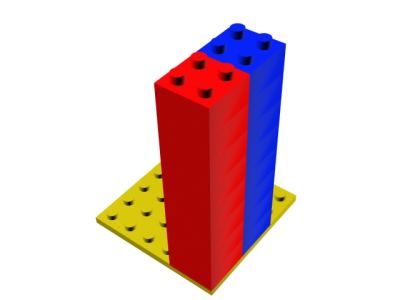
IMAGE - A simple analogy can be made between the stability of an atom and building blocks.
In this way no two protons or neutrons have the same energy and angular momentum as Pauli's principle stipulates is necessary. All of the blue blocks have to be in one stack and all of the red blocks in another. If there are too many nucleons the stacks simply get too high and become unstable. This limits the number of different elements that can be made by simply adding more and more nucleons. In fact the tallest stacks which together are completely stable correspond to 83 protons and 126 neutrons, this is an isotope of Bismuth and is the heaviest stable atom.
Please move mouse over image.
To explain why this maximum number of 126 neutrons cannot exist with just six protons to make a stable Carbon atom such that the strong nuclear force completely dominates the repulsive force of the 6 protons we have to realize that the range of distances over which the two forces operate are different. The repulsive electric force is felt throughout the nucleus. The strong nuclear force is very short range and acts only between immediate neighbours. There are only a certain number of neutrons that can get close enough to the protons to help hold them together, any extra neutrons simply make the neutron stack unnecessarily high. Atoms with taller stacks of neutrons and protons do exist but are inherently unstable and our building block analogy of these atoms will eventually begin to topple and result in radioactivity although in some instances this can in some instances this can take a very long time.
![]()

What is radioactivity?
We have seen above what contributes to a stable atom. Unstable nuclei will eventually decay and they do this by changing their internal structure. These are the radioactive nuclei and there are three distinct mechanisms by which they can become more stable - though not necessarily completely stable. These different modes of decay are named alpha, beta and gamma.
![]()
What is alpha particle decay?
We saw above that Bismuth 209 is the heaviest stable atom. Atoms which are heavier than this are forced to reduce their number of nucleons and they do this by ejecting them. It is not very easy to throw them out one at a time and it turns out that the easiest way to get rid of the excess in nucleons is to eject a pair of each at the same time. This ejected particle, containing two protons and two neutrons is exactly the same as a twice ionized Helium atom and is called an alpha particle. These are relatively heavy and slow moving so are easily stopped by thin paper. When alpha decay occurs the number of protons in the nuclei has changed and so the atom has become a different element. If the new element is also unstable it will decay further until it becomes a stable nuclei. An example of alpha decay occurs in Radium 226. This has 88 protons and in emitting an alpha particle it loses 2 protons, an atom with 86 protons is no longer Radium, it is Radon and its mass has reduced by four atomic mass units. The decay products of Radium 226 are Radon 222 and an alpha particle.
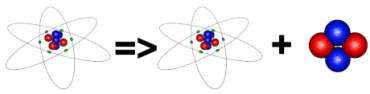
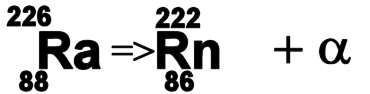
Radium-226 is a heavy nuclei and is unstable against alpha decay. It has a half life of around 1600 years. Radium-226 nuclei eject an alpha particle and become Radon-222 nuclei. Radon-222 is also unstable and will decay further.
![]()
What is beta particle decay?
A second way for nuclei to alter their internal structure is via Beta decay and this occurs in atoms where there is a significant disparity between the numbers of protons and neutrons. It usually involves neutrons turning into protons to even up the numbers but in a small number of nuclei it is necessary for protons to turn into neutrons. Consider as an example an atom of Boron 12. This has 5 protons and 7 neutrons and the energetics of this arrangement make it unstable. However, if one of the neutrons changed to a proton it would become Carbon 12, with 6 of each nucleon, this is more stable. It is surprising that neutrons can simply change into protons when necessary and it requires the operation of an entirely new fundamental force, the weak nuclear force. However, it is not just a simple one for one conversion, whilst protons are charged, neutrons are not and the need to conserve electric charge forbids indiscriminate swapping between the two. When the Boron 12 atom changes to Carbon 12 the universe has gained the extra charge of a proton. To cancel this out the reaction also needs to create the same magnitude negative charge and it does this by creating and ejecting an electron - this is how some of the excess energy in the unstable configuration is removed from the atom. This is emitted from the atom and is the particle we detect as beta radiation. Beta particles are light and typically move very fast, they will pass through paper but are stopped by thin sheets of metal. This is still not the full story of beta decay, the universe also has to conserve the general types of particle it contains. By spontaneously creating an electron it suddenly has one too many of a family of particles called leptons and the beta decay process also has to address this. It does so by producing a particle from the family which is the opposite of leptons and this particle is called an anti-neutrino. The Boron 12 atom decays to a Carbon 12 atom plus an electron plus an anti-neutrino.
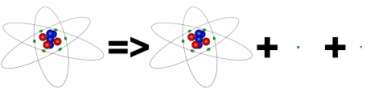

Boron-12 has an excess of neutrons above the most efficient way to pack 12 nucleons. Accordingly it decays by converting one of it's neutrons into a proton to become Carbon-12. To balance charge and lepton number it must eject a beta particle (now known to be an electron) and an anti-neutrino. It has a very short half life of 0.02 seconds.
![]()
What is gamma radiation?
Sometimes particular nuclei could be stable if they were in a particular configuration but are badly packed. These nuclei can become more stable by re-arranging themselves and getting rid of some of the extra energy they contain. This energy is carried away by a very energetic photon which is known as gamma radiation. There are a fixed number of configurations for any given nuclei and this means that in re-arranging themselves they release fixed amounts of energy. A sample of a particular material which is unstable against gamma decay will emit fixed energy photons.
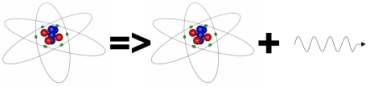
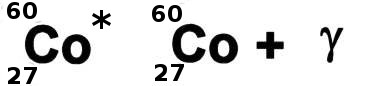
Cobalt-60 can exist in a state with its protons and neutrons inefficiently packed. It can re-arrange itself and release energy so becoming more stable. The energy is released by electro-magnetic radiation which is very energetic compared to visible light and called gamma radiation. Cobalt-60 has a half life of 5.3 years.
![]()
If some atoms are unstable how do they come to exist?
Unstable nuclei can be made so long as there is enough energy available to force together the unstable configuration of nucleons. This can happen in natural processes, and in particular occurs with the intense conditions associated with the gravitational collapse of a star - known as a supernova, or in a laboratory where for instance firing high energy neutrons into some atoms can cause them to adhere to the nucleus and create new isotopes. The question is how long does it take for the newly created unstable atom to decay back to a stable one. For any particular atom of an unstable element the radioactive decay is a random process but we can determine an average time for a whole collection of atoms. For a very unstable nuclei it might only take, on average, a tiny fraction of a second, these are the sort of atoms we are unlikely to see in nature. For atoms which are only a little unstable the average time for a decay to occur can be enormous, comparable to the age of the universe. To characterize the time scales of decay we determine the average time after which half of a collection of atoms will have decayed. This time is called its half-life. If there are 1000 radioactive atoms and in 2 hours half of them have decayed so we have 500 radioactive atoms and 500 of the decay product atoms then the half life for that particular nucleus is 2 hours. In the next 2 hours roughly half of the remaining 500 radioactive nuclei will have decayed leaving 250 radioactive nuclei and 750 decay product atoms. It is important to note that the strength of the source, ie the rate at which decays are occurring and hence particles ejected, falls exponentially and this explains how a radioactive source gets weaker with time (although this time can be so long we wouldn't notice) but will remain radioactive for very much longer than its half-life.
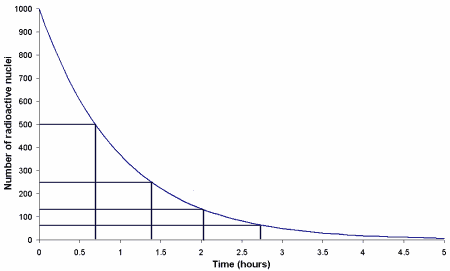
This plot shows how the number of radioactive nuclei in a sample falls with time. After 2 hours the original number of 1000 active nuclei has fallen to 500. The strength of the source has accordingly fallen in half. Of the remaining active nuclei a further half will have decayed in the following 2 hours leaving only 250 and so on down to 125 after 6 hours. The time taken for the strength of the source to fall in half is called the half-life and depends only on the particular element involved and exactly how unstable it is.
The End of the ATOMS topics.

